For
all
the
generative
AI
hoopla,
it
feels
as
though
the
legal
sector
remains
flummoxed
by
the
technology.
Everyone’s
talking
about
the
role
that
AI
will
play
in
the
industry,
but
hardly
anyone’s
actually
doing
anything
about
it
yet.
As
of
a
few
months
ago,
only
around
15
percent
of
Biglaw
had
taken
the
AI
plunge.
Meanwhile,
outside
of
the
legaltech
echo
chamber,
lawyers
are
much
more
likely
to
be
talking
about
GenAI
as
a
vector
for
malpractice
than
a
critical
tool
for
the
21st
century.
To
some
extent,
that’s
the
inevitable
byproduct
of
a
stuck-in-its-ways
legal
landscape.
But
technology
has
dragged
lawyers
kicking
and
screaming
into
the
future
before
and
it’ll
do
it
again.
Though
that’s
not
going
to
happen
until
GenAI
offerings
start
selling
lawyers
on
specific
points
on
the
workflow
where
the
technology
gives
us
something
that
a
supercharged
machine
can
deliver
better
than
throwing
more
humans
at
the
problem.
This
morning,
Thomson
Reuters
delivered
its
plan
to
capture
the
hearts
and
minds
of
the
industry,
announcing
a
single
GenAI
assistant
that
will
offer
applications
for
TR’s
range
of
professional
services
in
Legal,
Tax,
Risk
&
Fraud,
and
Media.
Customers
can
choose
which
services
they
need,
but
subscribing
to
more
will
allow
the
assistant
to
accomplish
more
—
critical
for
cross-disciplinary
work.
The
system
will
also
work
with
Microsoft
365
applications
allowing
users
to
call
upon
CoCounsel
directly
from
the
client’s
overly
needy
email
or
the
partner’s
borderline
unreasonable
Teams
message.
Let’s
look
at
it
in
action:
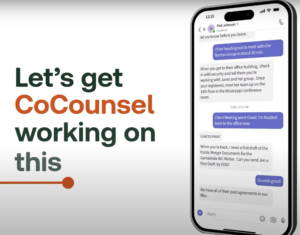
Now,
obviously
this
is
a
commercial,
but
that
looks
genuinely
useful.
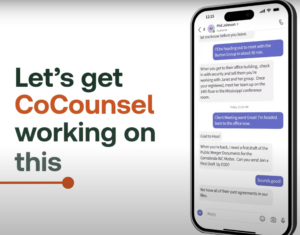
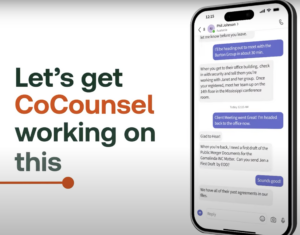
Breaking
it
down,
the
user
receives
an
assignment
over
Teams
chat
and
forwards
that
with
a
single
click
to
the
CoCounsel
app.
CoCounsel
reviews
the
chat
and
figures
out
what
the
partner
is
asking
and
figures
out
potential
assignments
that
it
can
perform
to
advance
the
ball
based
on
its
capabilities
and
the
Thomson
Reuters
knowledge
base
at
its
disposal.
Don’t
sleep
on
the
importance
of
a
tool
suggesting
its
own
use
cases.
The
biggest
obstacle
to
adoption
usually
rests
between
the
user’s
ears.
So
why
leave
it
up
to
the
lawyer’s
(lack
of)
imagination
to
figure
out
what
the
tool
can
accomplish?
There’s
a
reason
videogames
use
helpful
tips
as
loading
screens
—
people
will
try
new
moves
if
you
go
ahead
and
ask
them
to
give
it
a
shot.
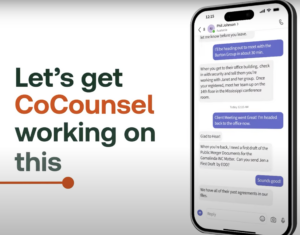
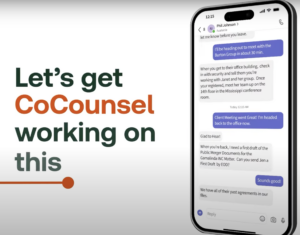
Then
the
system
goes
to
work
“all
before
you
are
back
at
the
office.”
Why
was
he
out
of
the
office
in
the
first
place?
You
can’t
expect
me
to
care
about
this
guy
without
doing
the
emotional
work.
Was
he
at
a
summer
lunch?
Is
CoCounsel
doing
the
heavy-lifting
while
our
protagonist
allows
those
three
martinis
wend
their
way
through
his
system?
I
need
backstory
here!
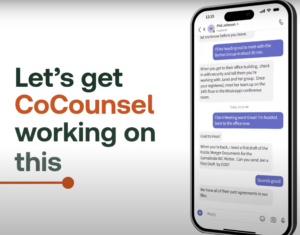
Moving
on,
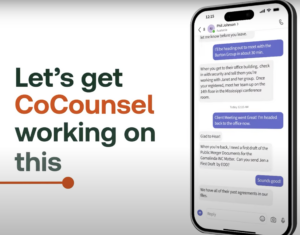
the
research
example
is
excellent.
I’ve
expressed
some
misgivings
about
the
power
of
GenAI
research.
It
can
certainly
review
the
caselaw
and
come
to
a
conclusion,
but
litigators
are
often
less
interested
today’s
“right”
answer
than
crafting
tomorrow’s
right
answer
—
one
that
draws
on
dissents
and
dicta
and
marginal
cases
to
extend
a
blanket
to
cover
the
client’s
novel
problem.
But
this
ad
shows
a
user
looking
for
guidance
on
breakup
fees
for
a
deal,
which
is
exactly
the
use
case
where
you’d
want
to
know
the
right
answer.
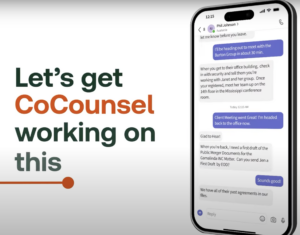
Easy,
one-click
links
to
see
the
results
in
Word
or
Practical
Law
makes
navigating
the
system
seem
simple
and
intuitive.
So
easy
that
he
has
time
to
look
longingly
out
the
window
of
the
worst
law
firm
conference
room
ever.
He
smiles
as
he
walks
outside
—
in
daylight!
—
having
delivered
for
the
client.
“Do
more
of
what
AI
can’t,”
it
says
as
he
looks
at
his
watch.
Presumably
to
check
if
he
has
time
for
another
martini.
Which
is
certainly
something
“AI
can’t.”
Earlier:
Westlaw
AI
Launch
Forces
Confrontation
With
The
Inner
Workings
Of
A
Lawyer’s
Mind
Maybe
We’ve
Got
The
Artificial
Intelligence
In
Law
‘Problem’
All
Wrong
 Joe
Joe
Patrice is
a
senior
editor
at
Above
the
Law
and
co-host
of
Thinking
Like
A
Lawyer.
Feel
free
to email
any
tips,
questions,
or
comments.
Follow
him
on Twitter if
you’re
interested
in
law,
politics,
and
a
healthy
dose
of
college
sports
news.
Joe
also
serves
as
a
Managing
Director
at
RPN
Executive
Search.